Disease Background
- 828,000 new cases of lung cancer and 657,000 of deaths from lung cancer[1] .
- 5-year survival rate for early-stage lung cancer is over 90%, and less than 10% for late-stage.
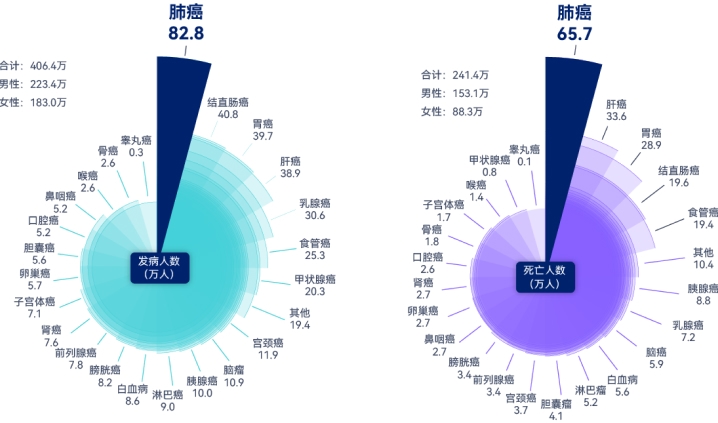

- Low detection rate of early lung cancer:In China, the detection rate of early lung cancer is 20%, the 5-year survival rate for lung cancer patients is 19.7%[1] and the detection rate of pulmonary nodules in health examination population is 22.6% [2].
- High false positive rate: The NLST research shows that the false positive rate of pulmonary nodules in LDCT detection is 96.4% [3].
- High incidence of anxiety: Research shows that the incidence of anxiety in the patients with pulmonary nodules is 59%[4].
- High excessive treatment rate:Of the nodules which are highly suspected to be malignant based on imaging and clinical judgment, 30%-40% are benign lesions after surgery[5].
Product Advantages

-
98.1%Sensitivity
-
91.7%Specificity
-
97.3%Accuracy
-
Noninvasive
Only 10 ml of peripheral blood and CT-DICOM image data are needed.
-
Professional
The results were published in the Journal of Clinical Investigation (2022 IF=15.9) and The Lancet Digital Health (2022 IF=30.8). PulmoSeek™ is CE Mark certified and recommended by the Chinese Expert Consensus on Early Lung Cancer Diagnosis (2023 edition). The benchmark has obtained ISO13485:2016 international certification
-
Intelligent National invention patent
NGS methylation detection intelligent analysis + image AI smart diagnosis, to assure stable performance.
-
Innovative
National invention patent, to ensure accurate detection, AnchorlRIS® library building technology related invention patents: methylation DNA detection method [patent No. : ZL20170063335.1], DNA ligase mediated DNA amplification technology [patent No. : 201710206888.8].

International Recognition
-
Thunder Project: To verify the performance of PulmoSeek Plus to distinguish benign and malignant pulmonary nodules in the real world
Academician Nanshan Zhong: "We hope to demonstrate that through clinical trials, AnchorDx's non-invasive relevant testing products can achieve early diagnosis and treatment of lung cancer in the real world for the benefit of patients, thus reflecting the socio-economic benefits in the entire health care system, as well as rewriting guidelines and becoming the gold standard"
-
American Society of Clinical Oncology
In 2023, Professor Liang Wenhua presented the latest research results of PulmoSeekTM Plus V2.0 at the American Society of Clinical Oncology
-
CE certification
In 2022, PulmoSeek™ Plus obtained IVDD-CE Mark from EU and an IVD European Access Permit from Dutch CIBG.
-
ERS International Congress
In 2022, Academician Nanshan Zhong presented the research findings of PulmoSeek™ Plus at the International Congress of European Respiratory Society (ERS).
-
World Conference on Lung Cancer (WCLC)
In 2021, PulmoSeek™ Plus was presented at the World Conference on Lung Cancer (WCLC).
-
Awards & Honors2021 Top 10 Excellent Total Solutions for Cancer


-
Awards & HonorsTop 10 enterprises in the "In Vitro Diagnostics High-Throughput Sequencing Top 10" category in China Smart Diagnostic Medicine List 2021


-
Awards & HonorsA unit of Guangdong Research Center for Precision Medicine and AI-Aided Diagnosis of TumorsGuangdong Tumor Precision Medicine and Artificial Intelligence Aided Diagnosis Engineering Technology Research Center


Target Users
Patients with 5-30mm pulmonary nodules
-

Initial CT scan revealed an uncertain lung nodule that was difficult to diagnose on 5-8mm imaging
-

Patients with 5-30mm solid/subsolid nodules found on CT at first visit seek scientific management
-

During the follow-up period, the nodules showed certain characteristic changes, and it was necessary to distinguish benign and malignant
-

Multiple pulmonary nodules requiring surgical decision making
Sample Collection and Service Process
-
10ml blood
- +
-
CT-DICOM data
-
 Test counseling
Test counseling -
 Sample collection
Sample collection -
 PulmoSeek™️ Plus test
PulmoSeek™️ Plus test -
 Report issuance
Report issuance -
 After-sales support
After-sales support
References
- [1] Report of Cancer Epidemiology in China, 2015. Chinese Journal of Oncology, 2019;41(1).
- [2] WenjiaYang, et al;[J],.Lung Cancer, 2018.Mar, 117,20-26.
- [3] NLST, [J].N Engl J Med,2011 August 4,365(5): 395–409.
- [4] Lihong Li, et al.[J].Thorac Cancer,2020 Jun,11(6):1433-1442.
- [5] Silvestri GA, et al. [J]. N Engl J Med,2015, 373(3):243-251.
- [6] JianXing He ,et,[J], The Lancet Digital Health. 2023; S2589-7500(23)00125-5
- [7] Chinese Society of Respiratory Medicine. Chinese expert consensus on early lung cancer diagnosis (2023 edition). Chinese Journal of Tuberculosis and Respiratory, 2023, 46(1) : 1-18. (in Chinese)





.jpg)






