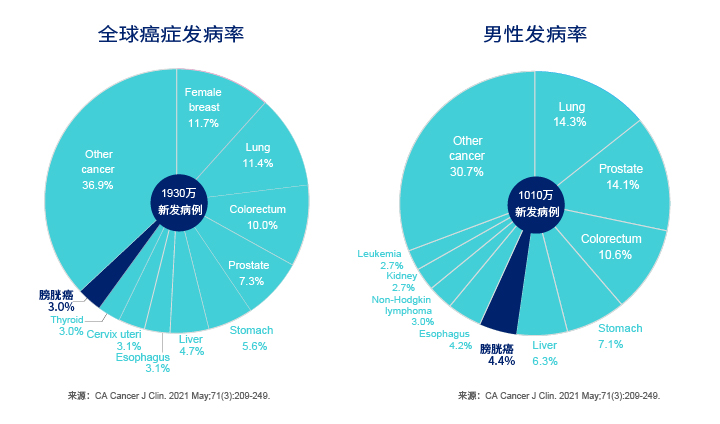
Disease Background
- High potential incidence: The data released by the American Urological Association and the data from the health examination centers in China show that the incidence of hematuria in the people receiving health examination is about 5%; and about 8% of the patients with hematuria have bladder cancer[1].
- The patients are getting younger: The disease can be seen in people at any age and shows a younger trend.

- Early screening significantly reduces the risk of death: The proportion (10%) of the patients who received early screening and ended up with high-grade bladder cancer is significantly lower than that (60%) of the patients who did not receive screening; none of the people who received early screening died of bladder cancer and 20.4% of the people who did not receive screening died of bladder cancer[2] .
- USA: The preventive medicine working group began in 2011 to recommend screening of bladder cancer in high-risk population[3].
- China: In 2020, Shanghai Anti-Cancer Association recommended screening of bladder cancer in high-risk population in the Recommendation on Screening and Prevention of Common Malignant Tumors in Residents[4].
Product Advantages

.jpg)
-
99%Accuracy of low-risk results >
-
Make up for omission
Bladder cancer test is usually not included in a health examination package. For UriInstantTM, a sample can be taken at home to easily detect bladder cancer.
-
Full coverage
From a bottle of urine, 3 kinds of urinary tumors can be detected, including bladder urothelial carcinoma, renal pelvis carcinoma and ureteral carcinoma.
-
Comfortable and convenient
With only 100ml of urine, the detection can be done, which is painless and convenient.
-
Stable performance
The performance is verified by nearly 20,000 cases of real-world test data to be excellent.

Professional Recognition
-
Inclusion in Guidelines
The detection method was included and recommended in the Chinese Guidelines for Diagnosis and Treatment of Urological and Andrological Diseases (2022).
-
Certification of US FDA
UriFind® earns "Breakthrough Device Designation" (BTD) from was identified as a breakthrough device (BTD) by U.S. FDA. The breakthrough technology has significant advantages and is in the best interest of patients compared with the existing products.
-
CE certification
UriFind® obtained European Access Permit for in-vitro diagnostics devices (IVDD) authorized by the Netherlands CIBG.
-
Patent recognition
The detection method was granted with a Chinese Invention Patent (patent No. ZL2019 1 1370095.5).
-
Awards & HonorsFirst Prize for Science and Technology Progress in Guangdong Province in 2021


Target Users
-

Chronic Cigarette smokers
-

People who perm and dye their hair frequently
-

People exposed to chemicals for a long time
-

People with a family history of urinary carcinoma
-

People with a history of other urinary diseases
-

People who take Chinese herbal medicine for a long time
Note: Women are not advised to take samples in the menstrual period and pregnancy
Sample Collection and Service Process
-
100ml of urine
-
 Ordering online/offline
Ordering online/offline -
 Sample Collection
Sample Collection -
 Sample Transport
Sample Transport -
 Testing and Analysis
Testing and Analysis -
 Report Issuance
Report Issuance -
 Report Interpretation Service
Report Interpretation Service
References
- [1] Ingelfinger J R. Hematuria in adults[J]. New England Journal of Medicine, 2021, 385(2): 153-163.
- [2] Messing E M, Madeb R, Young T, et al. Long‐term outcome of hematuria home screening for bladder cancer in men[J]. Cancer: Interdisciplinary International Journal of the American Cancer Society, 2006, 107(9): 2173-2179.
- [3]Ann Intern Med. 2011 Aug 16;155(4):246-51.
- [4]Shanghai Anti-cancer Association. Recommendation on Screening and Prevention of Common Malignant Tumors in Residents[J]. 2021







-569.jpg)
-35.jpg)












