High-standard Clinical Laboratory
AnchorDx Clinical Laboratory was established in 2016 in Guangzhou Ctiy, Guangdong Province, and obtaining the Practice License of Medical Institution in the same year. We were authorized to apply high-throughput sequencing technology to clinical applications in 2017. It is almost the first approved clinical laboratory in China.
The laboratory focuses on early screening and diagnosis of tumors using DNA methylation detection technology, covering lung cancer, bladder cancer, urothelium cancer, etc.

Qualification Certification

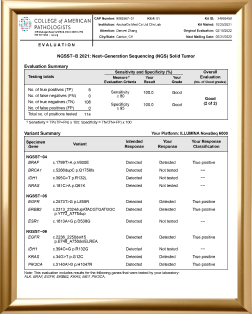
Evaluation of CAP Proficiency Testing


Acceptance Certificate of Clinical Laboratory
AnchorDx Clinical Laboratory has pass the proficiency testing organized by the National Center of Clinical Laboratories (NCCL) / the College of American Pathologists (CAP) for six years.