At the beginning of the New Year in 2024, Guangzhou AnchorDx Medical Co. Ltd. independently developed and produced the “Human ONECUT2/VIM Gene Methylation Test Kit (Fluorescent PCR Method)” (referred to as UriFind®). This new product received a Class III medical device registration certificate from the National Medical Products Administration (NMPA) (GXZZ20243400221) [1].
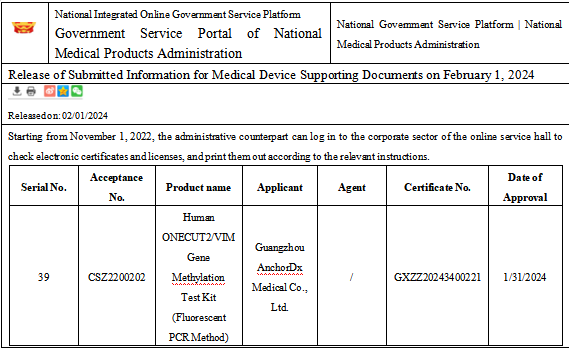
Human ONECUT2/VIM Gene Methylation Test Kit (Fluorescent PCR Method)
The "Human ONECUT2/VIM Gene Methylation Test Kit (Fluorescent PCR Method)" developed by AnchorDx Medical is the first test kit approved for the auxiliary diagnosis of urothelial cancer in China. This test kit is used for in vitro qualitative detection of the methylation levels of the ONECUT2 and VIM genes in human urine exfoliated cell samples. It is suitable for the auxiliary diagnosis of suspected urothelial cancer (including bladder cancer, ureteral cancer, and renal pelvis cancer) in newly diagnosed patients [2].
In the national multi-center registered clinical study of UriFind®, a total of 1,172 subjects were included in the statistical sample. The registered clinical study data shows that the test kit for the auxiliary diagnosis of urothelial cancer has a sensitivity of 89.74%, a specificity of 92.46%, and an accuracy of 91.47%, which enables comprehensive evaluation of bladder cancer, renal pelvis cancer, and ureteral cancer at one time. In addition, the UriFind® test kit has clear advantages in clinical use:
·Accuracy: It detects the methylation levels of 2 genes, with a clinical accuracy of 91.47% [2].
· Own patent: AnchorDx has obtained an invention patent for the test method (patent number ZL201911370095.5) [3].
· Recognition in the industry: The test kit is recommended by the 2023 edition of the "Diagnosis and Treatment Guidelines for Urology and Andrology Diseases in China" [4].
· Painless and convenient: 100ml random urine, non-invasive, painless, and convenient to collect samples [2].
Commercial Layout in China
With the approval and launch of the "Human ONECUT2/VIM Gene Methylation Test Kit (Fluorescent PCR Method)" by AnchorDx Medical, the company will fully initiate its commercial layout in China's market. This not only provides a new non-invasive clinical diagnostic tool for patients with urothelial cancer, but also offers a powerful weapon for early diagnosis and screening of urological tumors. AnchorDx Medical aims to improve the accessibility of products by occupying academic high grounds, gaining expert consensus and clinical guidelines, launching IVD products, conducting commercial operations, and building a comprehensive ecosystem for cancer diagnosis and treatment in the field of general health. This process involves the entire cycle from product research and development to production, which ultimately serves the purpose of benefiting the health and well-being of the people.
Global Market Layout
UriFind® by AnchorDx Medical has synchronized its registration in the United States. As early as July 2021, it has been recognized as "Breakthrough Device Designation, BTD" by the FDA, and has successfully initiated a prospective registered clinical trial (NCT05643690) in the US for the PMA application of UriFind® products (https://clinicaltrials.gov/study/NCT05643690), which lays the foundation for the global market layout of UriFind® products [5].
In the registration clinical study of UriFind® products in the United States, DiaCarta, as one of the clinical laboratory participants, has played a significant role, marking the beginning of the collaboration between AnchorDx Medical and DiaCarta in the United States. Furthermore, AnchorDx Medical has established a long-term partnership with DiaCarta in the United States. Both parties will collaborate on the development of cancer screening products and global commercialization [6].
As cancer has become a major and significant disease in China and around the world, early detection and diagnosis of cancer require a global strategic solution. With the registration application of UriFind® test kit in the United States and AnchorDx Medical's strategic collaboration with DiaCarta, it is poised to further accelerate the global expansion of early cancer screening.
[1] NMPA official website: https://www.nmpa.gov.cn/
[2] IFU for Human ONECUT2/VIM Gene Methylation Test Kit (Fluorescent PCR Method)
[3] Government Service Platform of China National Intellectual Property Administration http://ggfw.cnipa.gov.cn:8010/PatentCMS_Center/
[4] 2023 Edition of "Diagnosis and Treatment Guidelines for Urology and Andrology Diseases in China"
[5] Breaking News | AnchorDx Medical Launches U.S. Registered Clinical Trial of UriFind, an Early Bladder Cancer Detection Product, and Completes First Patient Enrollment
[6] AnchorDx Joins Hands with DiaCarta to Develop New Cancer Screening Products


